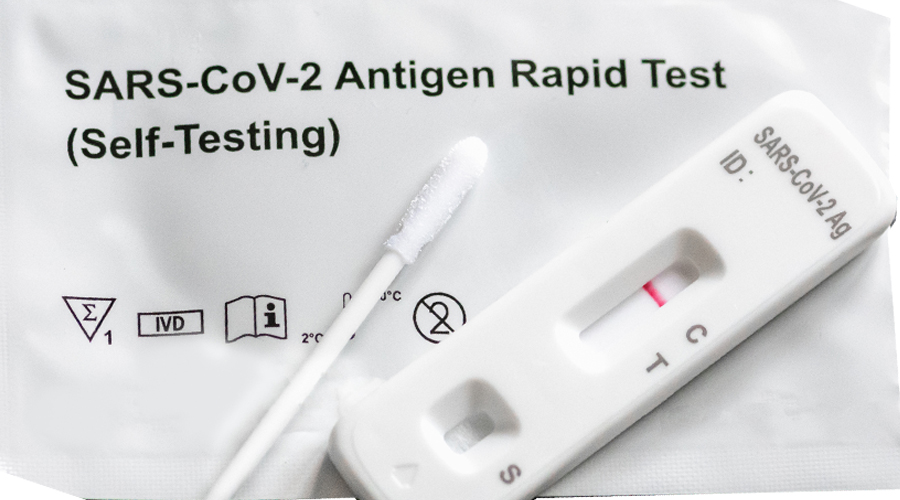
The health ministry along with the Drug Regulatory Authority (DRA) has approved the use of COVID-19 antigen self-test kits in the country. The ministry approved the emergency use of the test kit as an increasing number of people are able to access it. Pharmacies registered with the DRA will be allowed to sell or distribute self-test kits.
According to the health ministry, the approval of COVID-19 self-test kits comes following a thorough assessment.
However, individuals testing positive on self-test kits must report to 1010 for registration and monitoring by the clinical teams. The ministry will then facilitate immediate follow-up for vulnerable groups and monitor long COVID conditions.
Currently, there are around 60 COVID-19 testing centres across the country.
The DRA which regulates medicinal products in the country will be allowing authorized pharmaceutical firms to sell self-test kits. This is to ensure that the test kits are of reliable standard and good quality.
Authorized pharmacies importing the test kits must obtain an import authorization (IA) from the DRA.
As per the Medicines Act of Bhutan 2003, COVID-19 self-test kits fall under the category of medicinal products. It is classified as a Class D or high-risk medical device by Global Harmonization Task Force which promotes efficient and effective medical devices.
Kinzang Lhadon
Edited by Phub Gyem