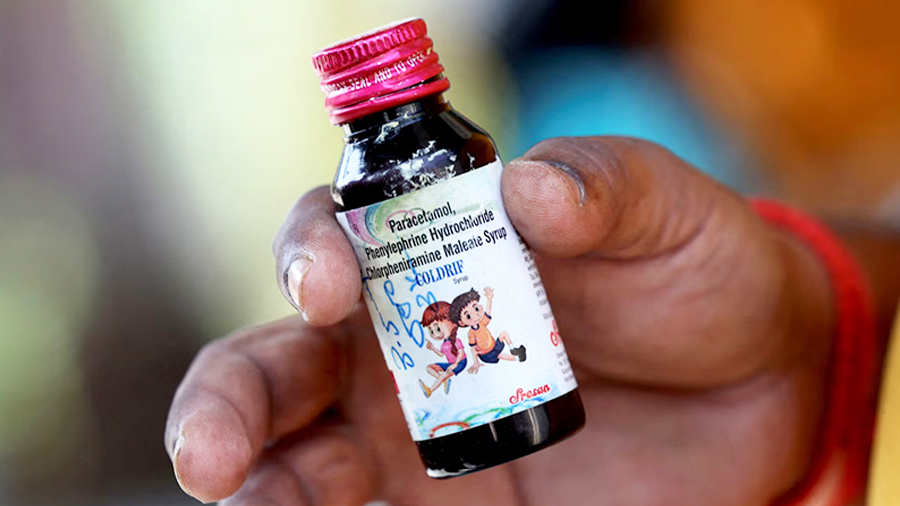
 Reports from India linking at least 20 deaths to a cough syrup brand called Coldrif have raised public concern about the safety and quality of medicines in Bhutanese pharmacies. This concern stems from the fact that most medical supplies, including cough and flu medicines, are imported from India. However, pharmacies in the country have assured that all cough syrups currently available in the market are safe. They also confirmed that Coldrif is not imported or sold in the country.
Reports from India linking at least 20 deaths to a cough syrup brand called Coldrif have raised public concern about the safety and quality of medicines in Bhutanese pharmacies. This concern stems from the fact that most medical supplies, including cough and flu medicines, are imported from India. However, pharmacies in the country have assured that all cough syrups currently available in the market are safe. They also confirmed that Coldrif is not imported or sold in the country.
According to Indian media, children who died after consuming cough medicine contained diethylene glycol, a toxic industrial chemical that should never be found in medicine. Kidney failure is common after consuming this poisonous chemical.
The deaths were all linked to Coldrif, banned after a test confirmed the presence of the chemical last week.
The health ministry and the Bhutan Food and Drug Authority have been contacted regarding the issue, and a response is awaited.
Meanwhile, pharmacies in the country have confirmed that all pharmaceutical imports are made through licensed dealers and monitored under national regulatory standards.
The Namsey Pharmacy in Thimphu, one of the country’s key pharmaceutical suppliers, said that all medicines entering Bhutan are regulated and undergo quality checks to ensure safety.
“Whenever we import from India, we have to apply for import authorisation. While doing so, if it is a cough syrup, not just cough syrup, any kind of syrup, we need to obtain a certificate, a lab-tested certificate for EEG and DEG specifically because this is the case. Even in India right now, the poisoning is because of DEG, diethylene glycol, which is used as a substitute for much more expensive products like glycerine and polypropylene glycol,” said Kinley Dorji, Competent Person, Namsey Pharmacy, Thimphu.
However, concerns remain over cross-border purchases of medicines, especially in border towns where people may buy over-the-counter drugs directly from shops, bypassing Bhutan’s regulatory system.
“If you go across borders and you ask for two tablets of antibiotics, like amoxicillin, they will just give you, because they don’t mind. Because for them, it is just to sell,” added Kinley Dorji, Competent Person, Namsey Pharmacy, Thimphu.
Indian health authorities have also advised people to avoid Respifresh and RELIFE syrups, made by companies in Gujarat, after the products were found to contain the same toxic chemical.
According to the World Health Organisation, India confirmed yesterday that the three contaminated syrups had been identified and none were exported.
Samten Dolkar
Edited by Sonam Pem