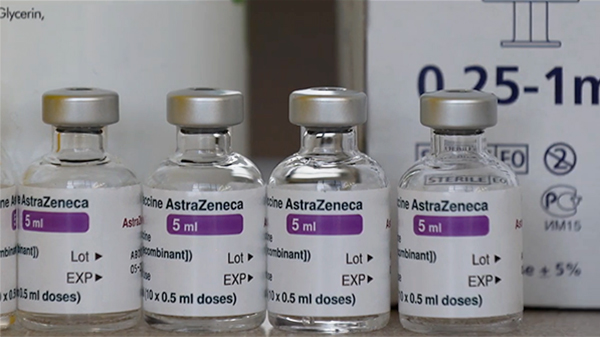
 The World Health Organisation (WHO) has approved Oxford-AstraZeneca vaccine for emergency use yesterday. The WHO is going to roll out vaccines to Low and middle-income countries through COVAX shared-procurement program. Around 170 countries are expected to receive their first deliveries of the vaccine at the end of this month.
The World Health Organisation (WHO) has approved Oxford-AstraZeneca vaccine for emergency use yesterday. The WHO is going to roll out vaccines to Low and middle-income countries through COVAX shared-procurement program. Around 170 countries are expected to receive their first deliveries of the vaccine at the end of this month.
According to a press release from the WHO, the organisation approved the vaccine produced by AstraZeneca-SKBio in the Republic of Korea and the Serum Institute of India. The vaccine met the required criteria for safety and its effectiveness outweighed its risks.
The vaccine is the second product to be added to the WHO’s list of COVID-19 vaccine after Pfizer vaccine.
The WHO hopes to deliver more than 330 million doses in the first half of the year and up to 2 billion by the end of December.
“We now have all the pieces in place for the rapid distribution of vaccines. But we still need to scale-up production, and we continue to call for vaccine developers to submit their dossiers to WHO for review at the same time as they submit them to regulators in high-income countries,” said Dr Tedros Adhanom Ghebreyesus, the Director-General of WHO.
Officials with WHO said two doses of the vaccine will be given to adults with an interval of around eight to 12 weeks.
More than 109 million people are infected by Coronavirus globally. Infections were reported in more than 210 countries since the first cases were identified in China in December 2019.
Sangay Chezom